Publishing
Aquila Solutions has over 40 years combined experience working with biologic and pharmaceutical companies as a consultant and publisher for their submissions to the FDA.
Biologics and Pharmaceuticals:
Working With Clients to Speed Up Submissions
Aquila offers the following publishing services:
eCTD Publishing
- Aquila’s in-house staff prepare client documents for submission. While our processing team handles all document processing activities, our publishing staff create the XML backbones for a variety of eCTD submissions, such as INDs, NDAs, DMFs, and BLAs. We publish in both DTD 2.01 and 3.3.
- Our teams can prepare most submissions with a same-day or next-day turnaround.
SPL Authoring
- Aquila’s publishing staff includes a specialized labeling team, allowing the creation and validation of FDA-compliant labels, such as over-the-counter and prescription drug labels, often in a matter of hours.
- Aquila processes all SPL types, including drug labels, establishment registrations, labeler code requests, and no-change notifications.
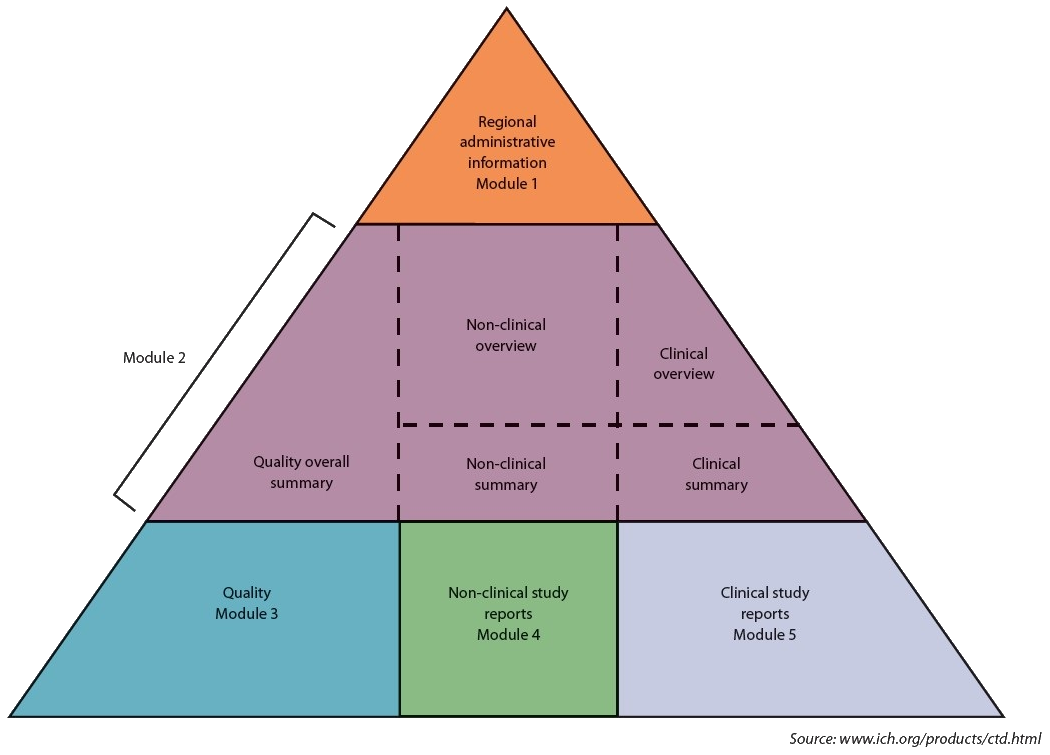
eCTD Structure
Get In Touch
Document Editing
- Aquila has encountered some submissions with unsatisfactory formatting and editing. Documents with mislabeled sections, tables and their titles being on separate pages, and other errors can occur while translating data into documents. Aquila offers full document revision, tracking all changes so the client can make the final decision on all revisions.

eCTD Structure
For more information about who we are and what we can do for your submission, please contact us!